How to use OXERVATE®
The way OXERVATE® is prepared and applied may be a change for you. This dosing and administration overview will help you understand how to use OXERVATE.

Learn how to dose and apply OXERVATE properly
Applying OXERVATE is a multistep process using the materials in the Delivery System Kit and should only be done according to the Instructions for Use.
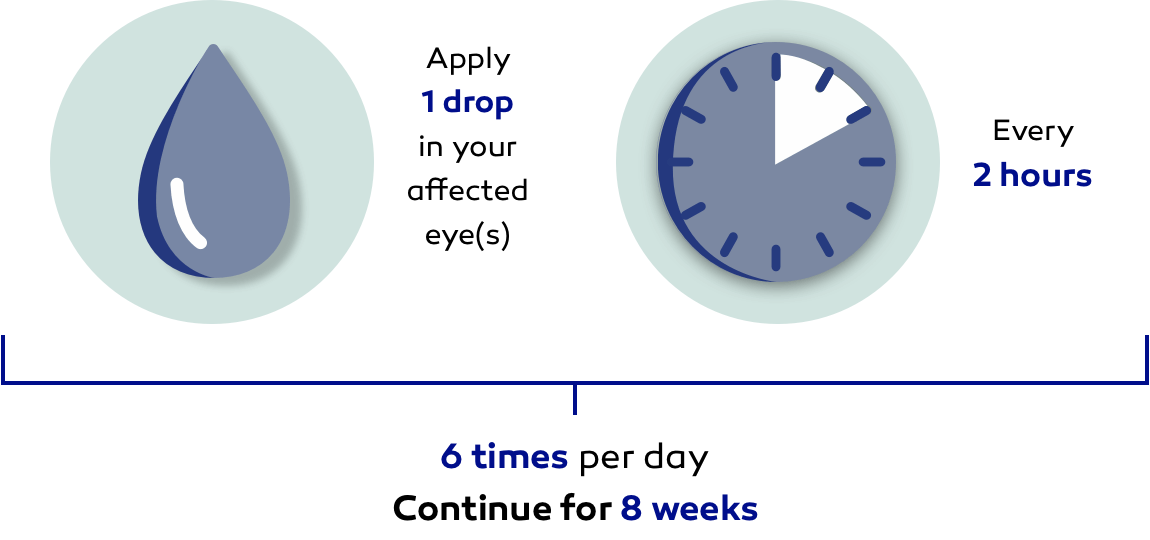
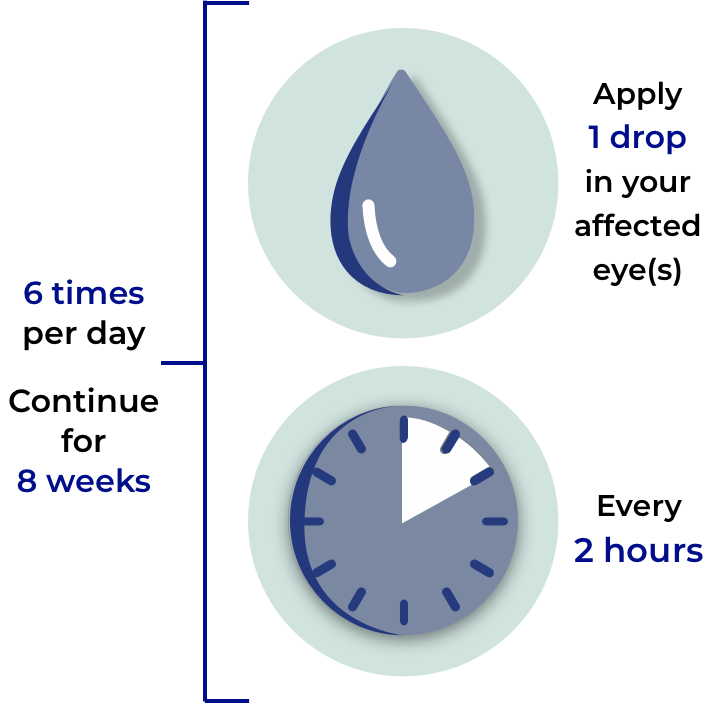
Follow your daily dosing schedule:
It is extremely important that you use OXERVATE exactly as your doctor tells you and complete the full course of treatment for it to be effective.
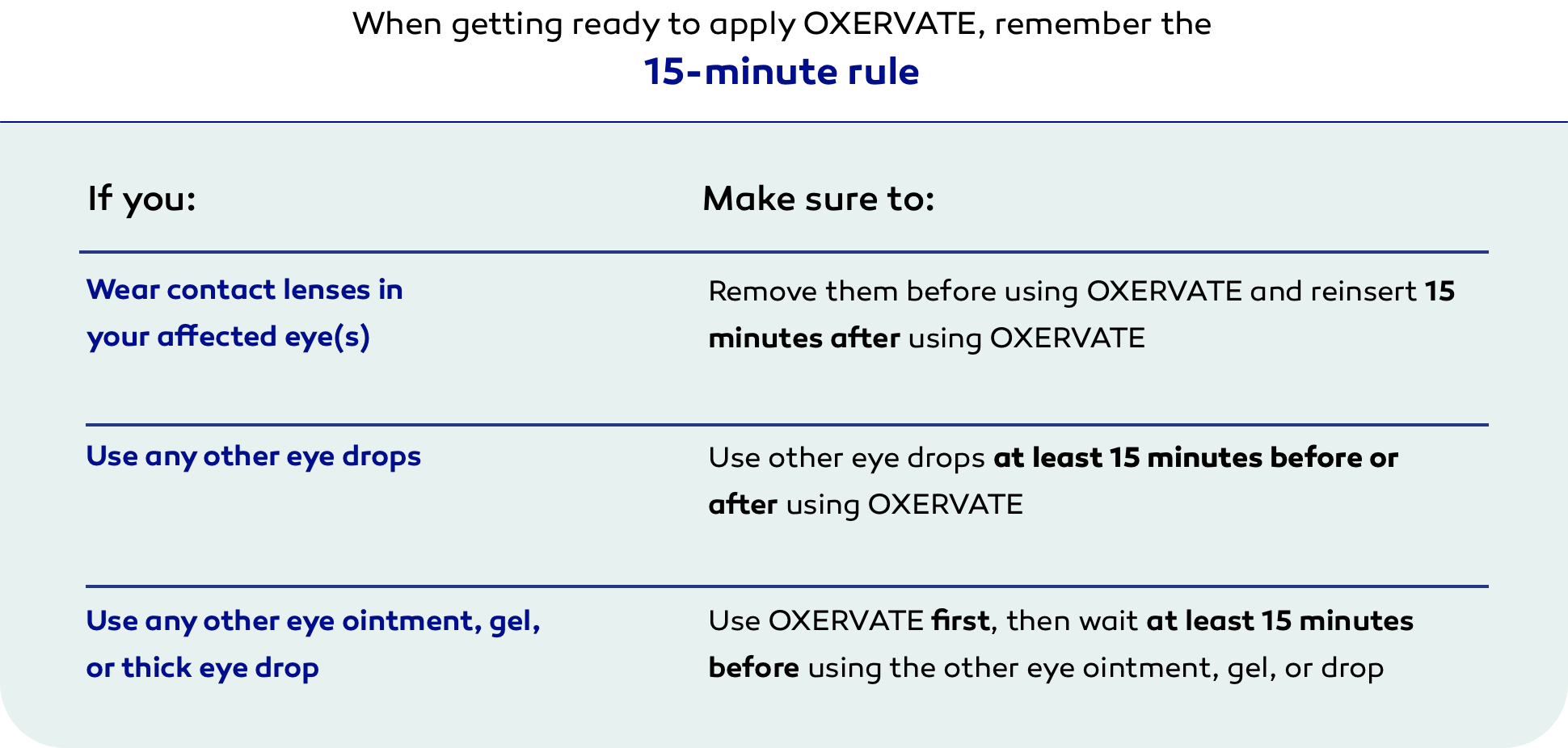
When getting ready to apply OXERVATE, remember the
15-minute rule
If you wear contact lenses in your affected eye(s)
- Make sure to remove them before using OXERVATE and reinsert 15 minutes after using OXERVATE
If you use any other eye drops
- Make sure to use other eye drops at least 15 minutes before or after using OXERVATE
If you use any other eye ointment, gel, or thick eye drop
- Make sure to use OXERVATE first, then wait at least 15 minutes before using the other eye ointment, gel, or drop
If you miss a dose of OXERVATE, take your next dose at your scheduled time and do not take an extra dose to make up for the missed one.
Do not use other eye medicines without talking to your doctor.
How to use
A biweekly delivery of OXERVATE includes everything you’ll need for 2 weeks of administration: 2 weekly cartons, each with 7 multiple-dose vials of OXERVATE, and 2 Delivery System Kits, each with 7 days’ worth of supplies. Patients with NK in both eyes will receive 4 weekly cartons in an insulated pack and co-packaged with 4 Delivery System Kits.
OXERVATE
weekly carton


7 multiple-dose vials of OXERVATE
Delivery System Kit

7 vial adapters (+1 extra)
42 pipettes (+3 extra)
42 sterile
disinfectant wipes (+3 extra)
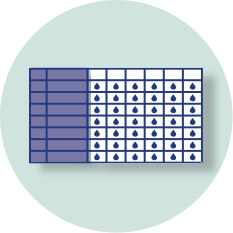
Use the Dose Recording Card included in your Delivery System Kit to:
- Track your 6 doses per day
- Write down the date you start a new weekly supply
- Note the time you opened a vial (when you connect the vial adapter to the vial during the week)
- Track your 6 doses per day
- Write down the date you start a new weekly supply
- Note the time you opened a vial (when you connect the vial adapter to the vial during the week)
Connect with us
Sign up to receive more information, including tools and resources for accessing and using OXERVATE.
By clicking SIGN UP, you agree to our Terms of Use and Privacy Policy.