Take a
closer look
at OXERVATE®
When your doctor prescribes OXERVATE® you’ll be supported every step of the way—from accessing treatment and receiving deliveries, to learning how to properly prepare and apply OXERVATE.



The only prescription eye drop FDA-approved to treat neurotrophic keratitis (NK).
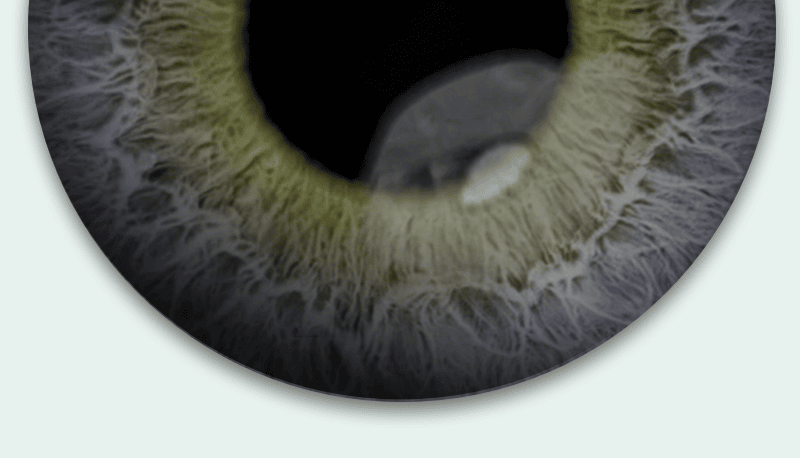
What is neurotrophic keratitis?
Neurotrophic keratitis, or “NK,” is an eye disease that may be caused by damage to the corneal nerves. This damage may lead to poor healing and degeneration of the cornea. If not treated, it may lead to a corneal ulcer (an open sore in the cornea).
This image is for illustrative purposes only.

Preparing for treatment
This dosing and administration overview will help you understand how to use OXERVATE.
Connect with us
Sign up to receive more information, including tools and resources for accessing and using OXERVATE.
ALL FIELDS REQUIRED
