What is OXERVATE®?
The only prescription eye drop FDA‑approved to treat people with neurotrophic keratitis (NK).
Our Terms of Use and our Privacy Policy have been updated. We strongly encourage you to read them as your continued use of our website indicates your acceptance of these policies.
The only prescription eye drop FDA‑approved to treat people with neurotrophic keratitis (NK).


Cenegermin-bkbj is a manufactured protein that is identical to a protein your eye creates naturally. This protein is called nerve growth factor (NGF).
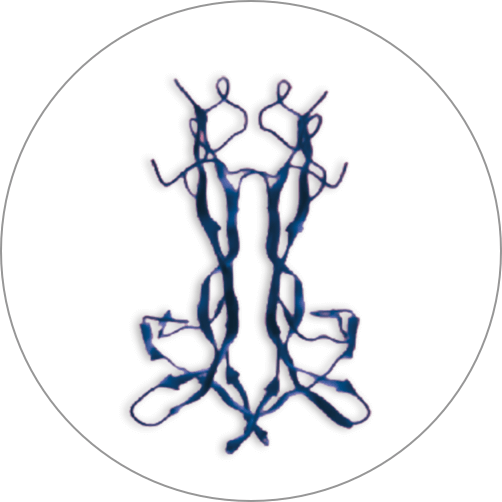
Cenegermin-bkbj manufactured
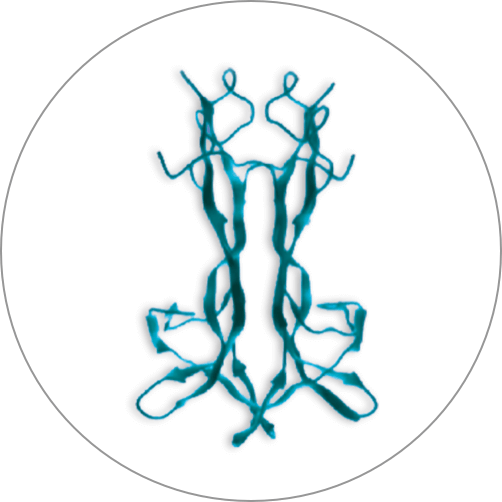
Nerve growth factor (NGF) made in your body
In a healthy eye, NGF helps your cornea and corneal nerves stay healthy.
NGF plays an important role in the development and survival of nerve cells
In the clinical trials, patients with NK were treated with OXERVATE® or placebo for 8 weeks
After 8 weeks, each patient’s degree of healing was determined by a doctor by staining the cornea. If there was no staining in the damaged part of the cornea, and no persistent staining in the rest of the cornea, the cornea was considered completely healed.
At a follow-up visit 48 weeks after the treatment period, patients in the REPARO trial who were completely healed were evaluated by a doctor again. If staining in the cornea was less than 0.5 mm in size, the cornea was considered to have remained completely healed.
Doctors use corneal staining to assess the amount of corneal tissue damage.
The greater the amount of stain, the more tissue damage.
In REPARO (Study NGF0212), 101 patients with NK in 1 eye were evaluated.
In Study NGF0214, 47 patients with NK in 1 or both eyes were evaluated.
You may experience side effects while using OXERVATE®.
Ask your doctor what side effects you may experience when starting your treatment with OXERVATE

If you’re prescribed OXERVATE you may not know what to expect or feel uncertain about the process. Use these sample questions to help start a conversation with your doctor and get the information you need to feel confident using OXERVATE.
Download the OXERVATE Brochure and use the Doctor Discussion Guide within during your next appointment
Sign up to receive more information, including tools and resources for accessing and using OXERVATE.
By clicking SIGN UP, you agree to our Terms of Use and Privacy Policy.
What is OXERVATE?
Before you use OXERVATE, tell your doctor about all of your medical conditions, including if you:
How should I use OXERVATE?
What should I avoid while using OXERVATE?
What are the possible side effects of OXERVATE?
Tell your doctor if you have any side effects that bother you. These are not all the possible side effects of OXERVATE.
For more information about OXERVATE talk to your healthcare provider or pharmacist.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1‑800‑FDA‑1088. You can also contact Dompé U.S. Inc. at 1‑833‑366‑7387.
Please see full Prescribing Information and Patient Information for OXERVATE.