NK is caused by corneal nerve damage1
Corneal nerve damage may lead to a decrease in or total loss of corneal sensitivity—the hallmark of neurotrophic keratitis (NK). This nerve damage impacts ocular surface homeostasis by disrupting the interplay between corneal nerves and corneal epithelial cells, which can lead to epithelial breakdown, including ulceration and, in severe cases, melting and perforation.1
Etiologies
Any injury or systemic condition affecting corneal sensory innervation can lead to NK1-5
Ocular
- Post-herpetic infection
- Ocular surgery (eg, LASIK, cataract surgery, corneal transplant, vitrectomy)
- Chronic ocular surface inflammation or injury
- Contact lens wear
- Chronic dry eye disease
- Topical ophthalmic drug toxicity
- Chemical and physical burns
Systemic
- Diabetes
- Multiple sclerosis
- Vitamin A deficiency
- Leprosy
- Amyloidosis
CNS
- Post-neurosurgical procedures
- Stroke
- Neoplasm
- Aneurysms
- Degenerative CNS disorders (eg, Parkinson’s)
Genetic
- Riley-Day syndrome
- Goldenhar-Gorlin syndrome
- Moebius syndrome
- Familial corneal hypoesthesia
A patient with 1 or more of these conditions or etiologies should prompt suspicion of neurotrophic keratitis (NK)1,2,4,5
Presentation & Signs
Patients with NK may not complain of symptoms due to decreased corneal sensitivity2
Signs and symptoms of neurotrophic keratitis (NK) can include2:
Dryness
Reduced blinking
Photophobia
Blurry vision
Stages
NK is classified into 3 stages of increasing severity*2
Neurotrophic keratitis (NK) is progressive—and if left untreated can lead to severe corneal damage6
defect (PED)2
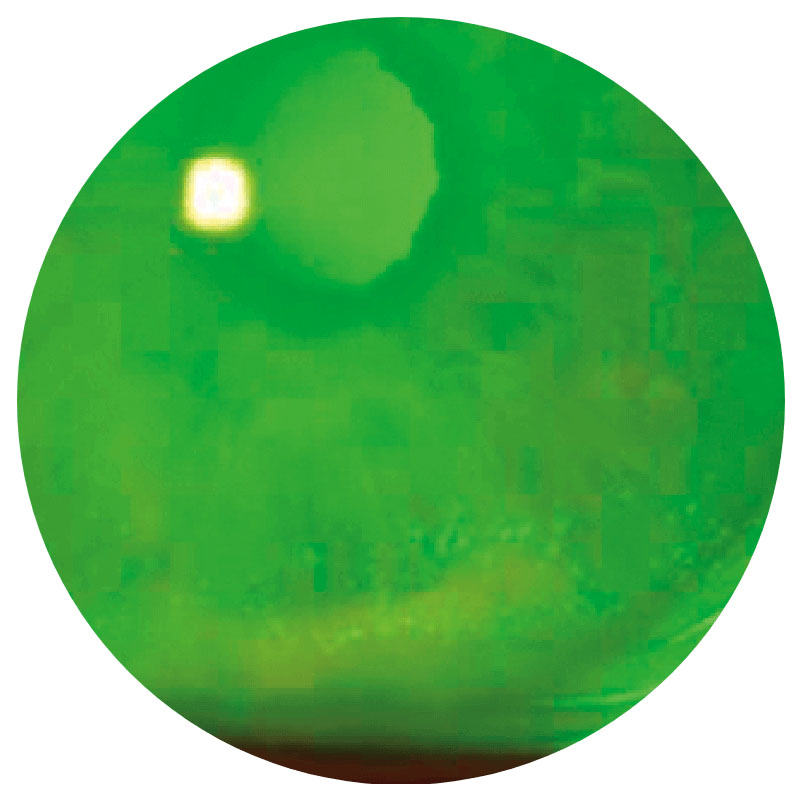
Stage 1
Punctate epithelial keratitis (PEK)Surface epitheliopathy2,7
Stage 2
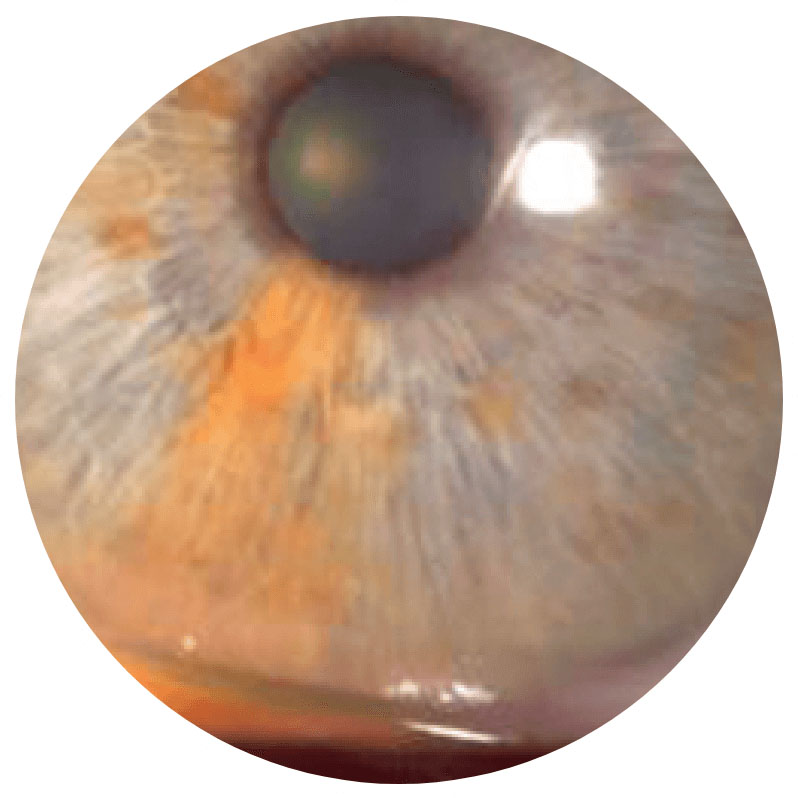
Persistent epithelialdefect (PED)2
Stage 3
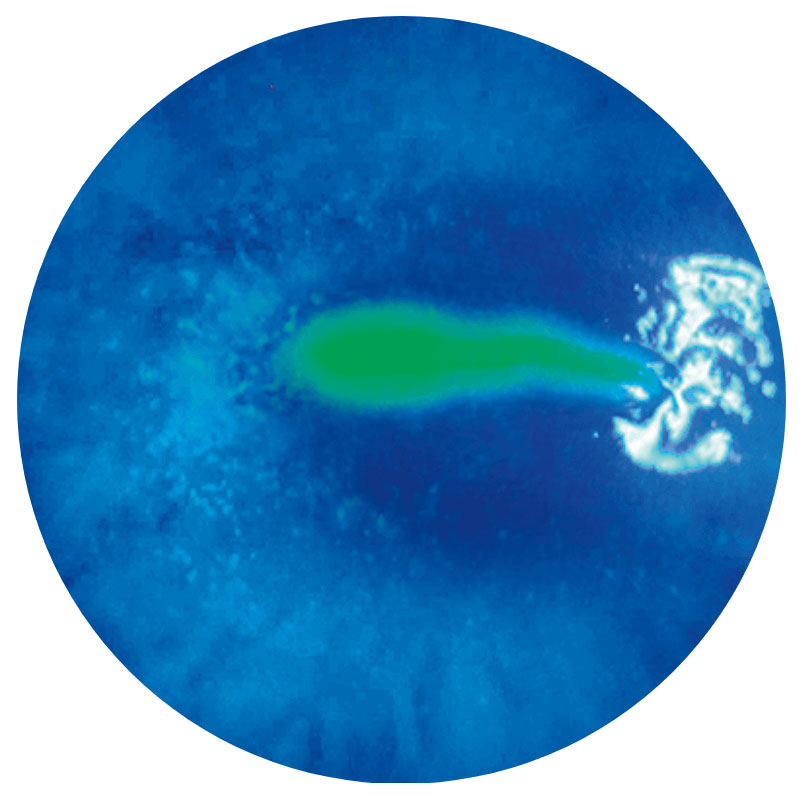
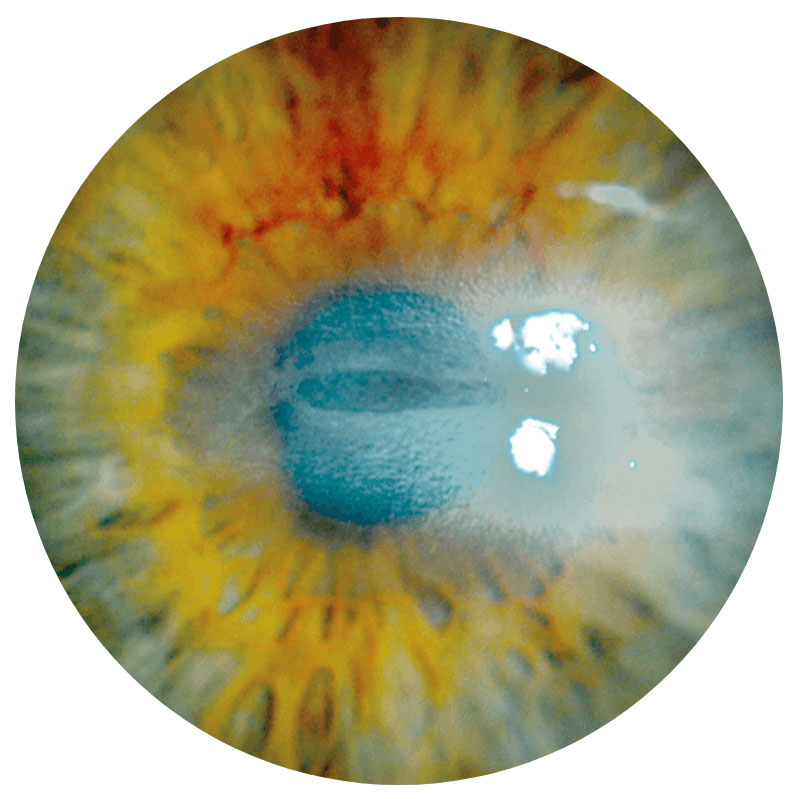
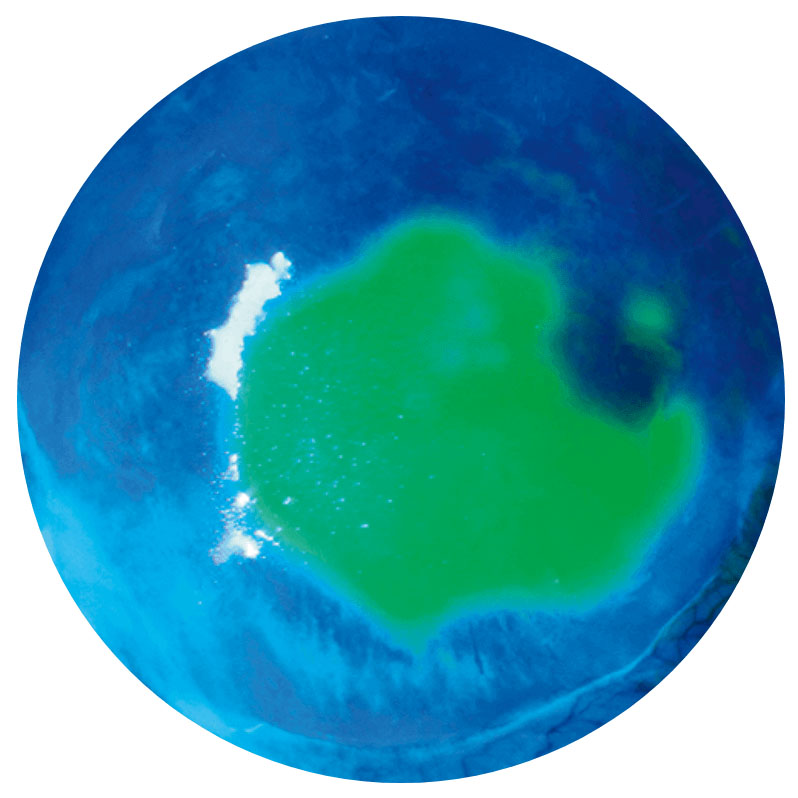
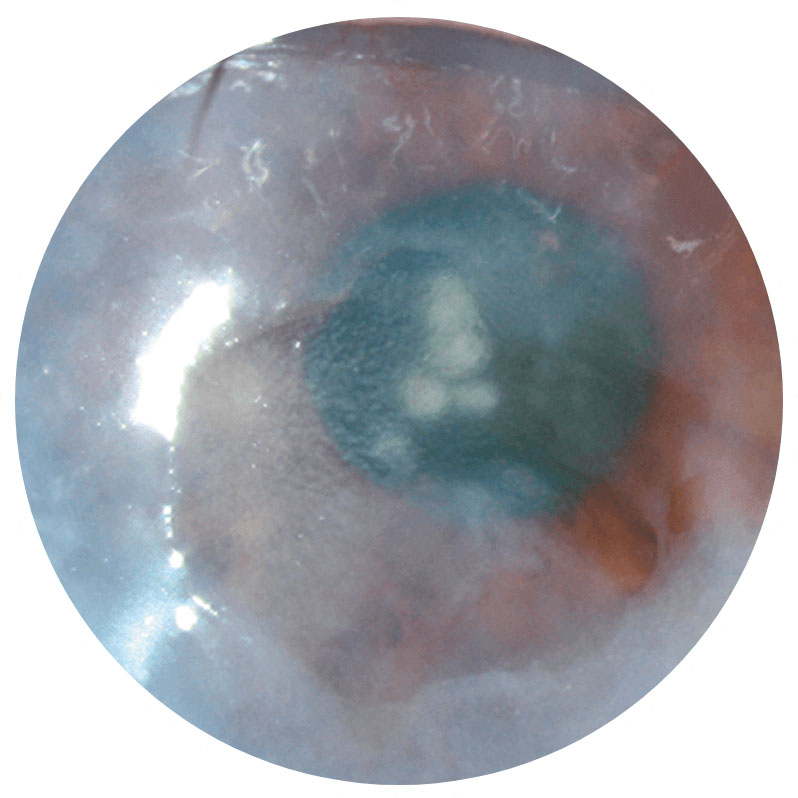
Corneal ulcer2NK is a progressive disease and can present at any stage—early diagnosis is essential2,4,6
* Based on the Mackie classification.2
Images show neurotrophic corneal lesions in 3 different patients and do not represent 1 patient’s disease progression.
Access the NK Diagnosis & Staging Tool
Diagnosis
When NK is suspected, confirm
with corneal sensitivity testing1
Early diagnosis is critical as progression of NK can often be asymptomatic.2,4,6
Consider corneal sensitivity testing as part of a broader diagnostic workup, including6:
- Patient medical history
- Microbiological exam
- Fluorescein staining
- Evaluation for systemic conditions
Important: Do not numb the eye when performing staining. Numbing will obscure results of corneal sensitivity testing.8
How to perform corneal sensitivity testing2,6
- Qualitative testing: cotton wisp or dental floss
- Quantitative testing: Cochet-Bonnet esthesiometer
- Test all 4 quadrants and the center of the cornea
- Record sensation as normal, reduced, or absent using the healthy eye as reference