in patients with NK3,4
course of therapy1
single point of contact

About NK
NK may be caused by corneal nerve damage5
Corneal nerve damage may lead to a decrease in or total loss of corneal sensitivity—a hallmark of neurotrophic keratitis (NK).6
This impairment in corneal innervation and sensitivity may then lead to epithelial breakdown in the cornea.6

MECHANISM OF ACTION (MOA)
The active ingredient in OXERVATE is a recombinant human nerve growth factor (rhNGF)1
Nerve growth factor (NGF) is an endogenous protein involved in the differentiation and maintenance of neurons, which acts through specific high-affinity (ie, TrkA) and low-affinity (ie, p75NTR) nerve growth factor receptors in the anterior segment of the eye to support corneal innervation and integrity.1
Efficacy & Safety
The efficacy and safety of OXERVATE for the treatment of NK were studied in a total of 151 patients, evaluated in two 8-week, randomized, multi-center, double-masked,
vehicle-controlled studies1
Patients received study treatment dosed 6 times daily in the affected eye(s) for 8 weeks.1
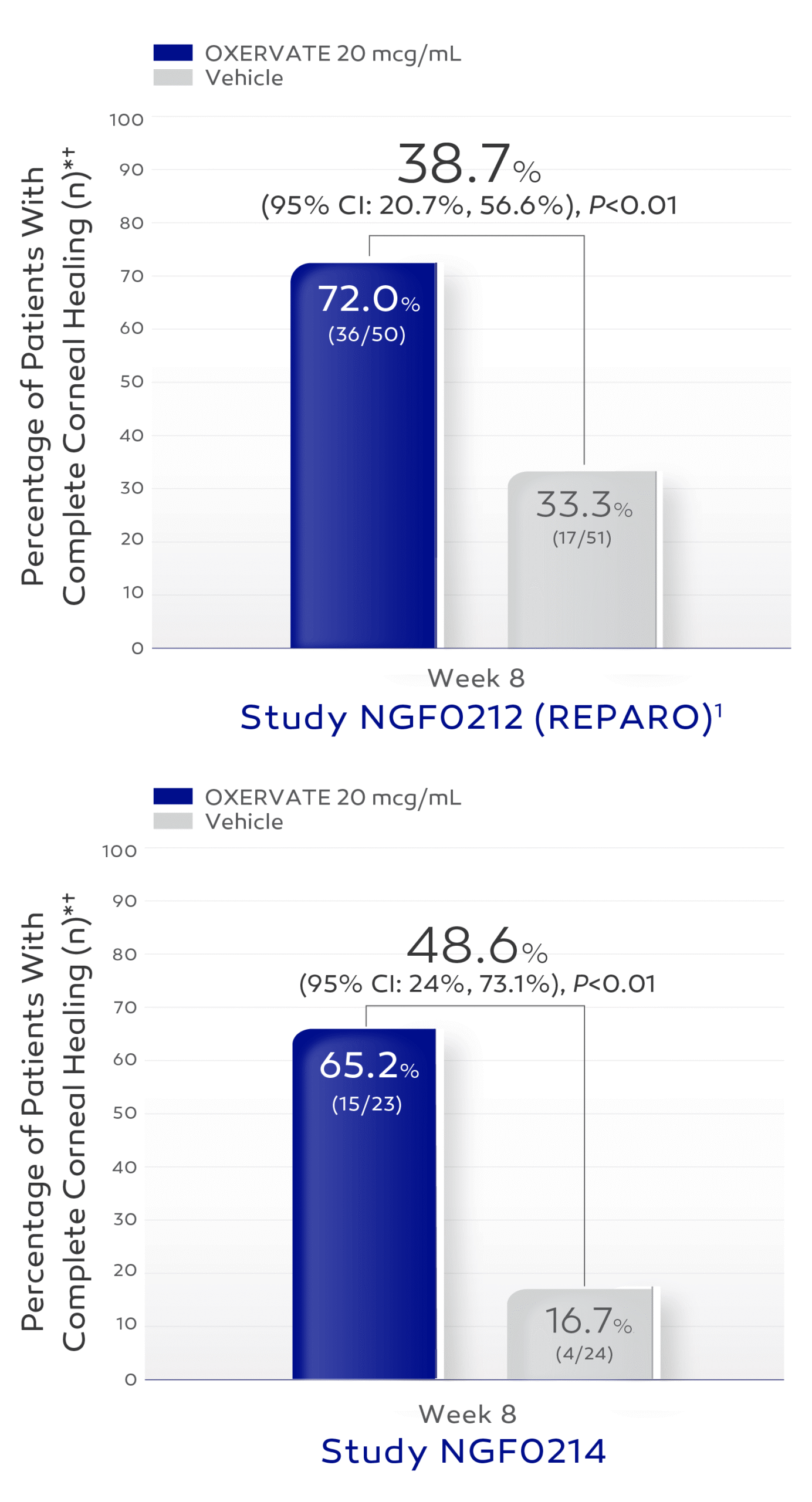
In clinical trials complete corneal healing was defined as absence of staining of the corneal lesion and no persistent staining in the rest of the cornea at 8 weeks of treatment.1
Patients without any post-baseline measurements were excluded from the analysis.1
In patients who were healed after 8 weeks of treatment with OXERVATE, recurrences occurred in ~20% of patients in Study NGF0212 and 14% of patients in Study NGF0214.1
OXERVATE safety in clinical trials1
The most common adverse reaction was eye pain following instillation, which was reported in approximately 16% of patients.1
Eye pain may arise as corneal healing occurs.1
Other adverse reactions occurring in 1% to 10% of OXERVATE patients included corneal deposits, foreign body sensation, ocular hyperemia, ocular inflammation, photophobia, tearing, and headache.1
Access support and more through a single point of contact
Dompé CONNECT to Care provides practices and patients with assistance every step of the way.





