This image is for illustrative purposes only.
NK may be caused by corneal nerve damage1
Neurotrophic keratitis (NK) is a rare degenerative corneal disease, which may be caused by trigeminal nerve impairment, resulting in a cascade of effects that may include a breakdown of the corneal epithelium.2
Damage to corneal innervation may lead to a decrease in or total loss of corneal sensitivity—a hallmark of NK.2
This nerve damage impacts ocular surface homeostasis by disrupting the interplay between corneal nerves and corneal epithelial cells, which may lead to epithelial breakdown, including ulceration and, in severe cases, melting and perforation.1,3
OXERVATE has not demonstrated clinically significant change in corneal sensitivity in clinical studies.4
This image is for illustrative purposes only.
Etiologies
Injury or systemic conditions affecting corneal sensory innervation may lead to NK2,5-7
Ocular
- Post-herpetic infection
- Ocular surgery (eg, refractive surgery, cataract surgery, corneal transplant, vitrectomy)
- Chronic ocular surface inflammation or injury (eg, dry eye disease)
- Contact lens wear
- Topical ophthalmic drug toxicity
- Chemical and physical burns
Systemic
- Diabetes
- Multiple sclerosis
- Vitamin A deficiency
- Leprosy
- Amyloidosis
CNS
- Post-neurosurgical procedures
- Stroke
- Neoplasm
- Aneurysms
- Degenerative CNS disorders (eg, Parkinson’s)
Genetic/Congenital
- Riley-Day syndrome
- Goldenhar-Gorlin syndrome
- Moebius syndrome
- Familial corneal hypoesthesia
PATIENT PRESENTATION
Patients with NK may not complain of symptoms due to decreased corneal sensitivity8
In the presence of more significant corneal involvement, patients with NK may experience blurry vision, while symptoms of ocular surface discomfort are uncommon.*8
*OXERVATE is not indicated for the treatment of the symptoms of NK.
CLINICAL PRESENTATION
NK presentation varies based on level of corneal damage2,9
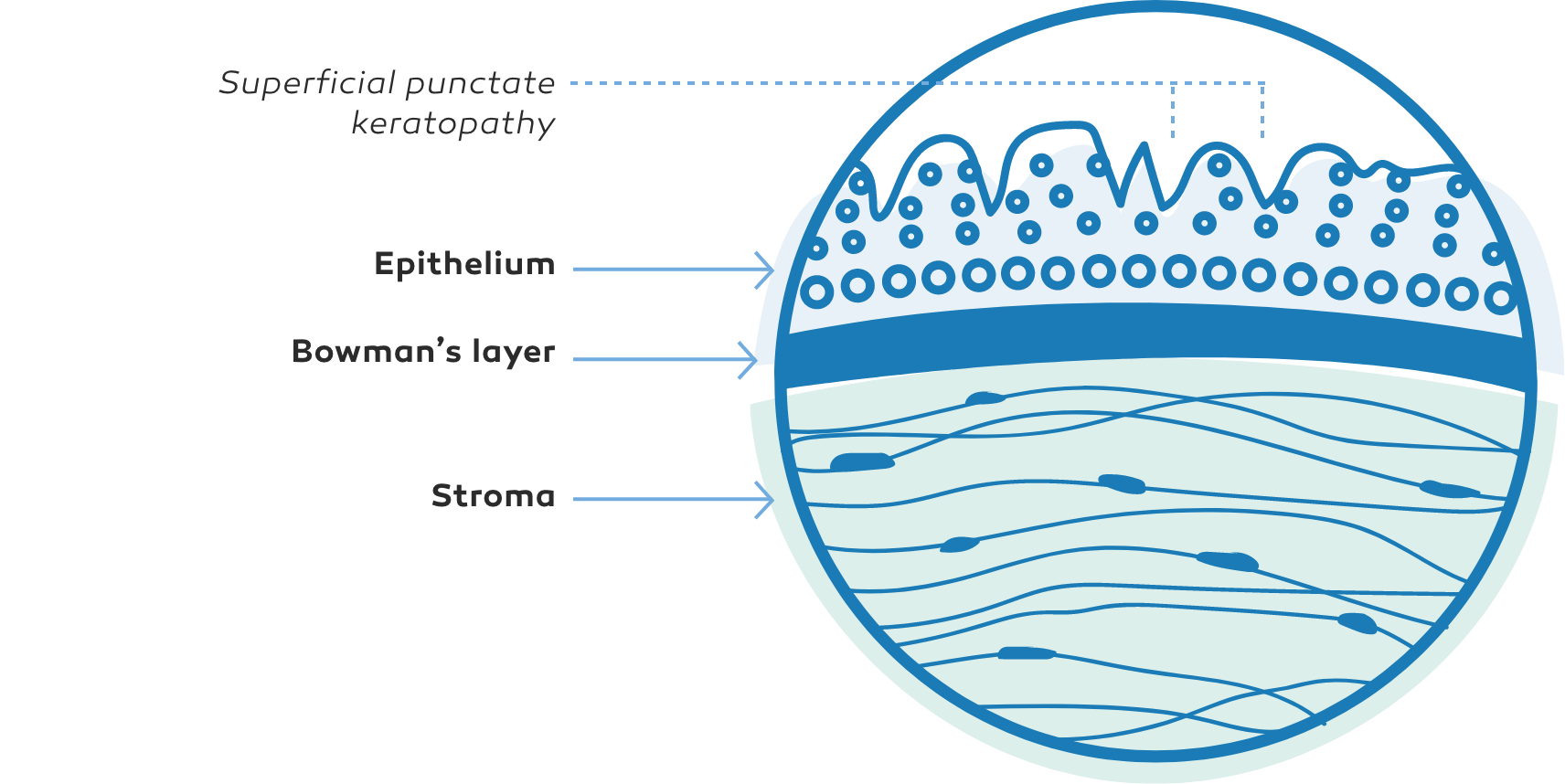
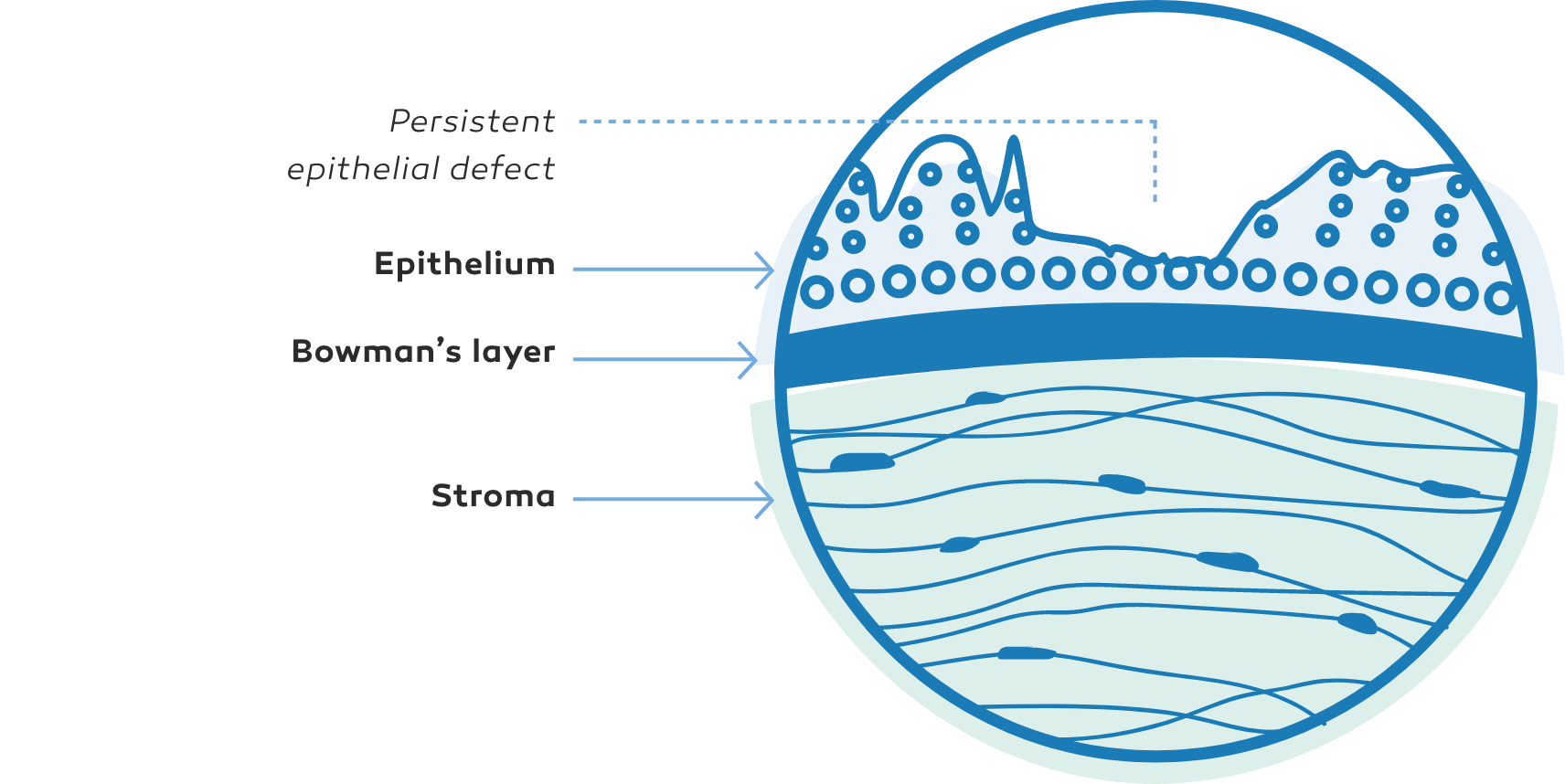
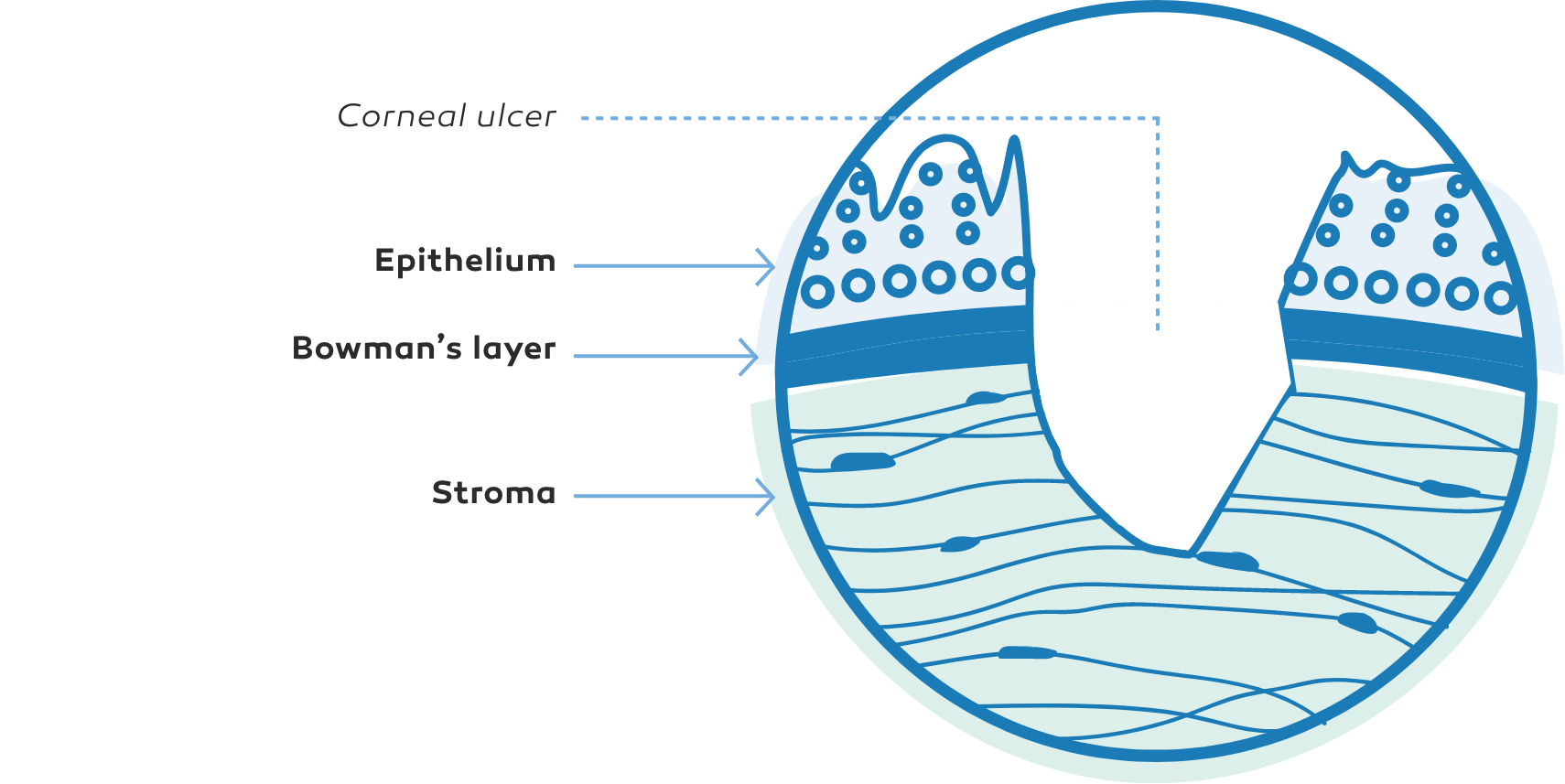
NK manifests as corneal epithelial changes ranging from superficial punctate keratopathy (SPK) to persistent epithelial defects (PEDs) and ulcers, which may progress to stromal melting and corneal perforation9
The Mackie classification system is a commonly used grading system for NK, separating the disease into distinct stages based on severity2,9
defect (PED)9
Stage 1
Superficial punctate keratopathy (SPK)9Stage 2
Persistent epithelialdefect (PED)9
Stage 3
Corneal ulcer9In some cases, NK may be a progressive disease2,6,9
The image is for illustrative purposes only. This is not an exact representation of a patient, or a structure and function.
Fluorescein staining may be useful in assessing corneal epithelial damage in NK2:
Based on the Mackie classification2:
- Punctate corneal epithelial fluorescein staining
- Decreased tear breakup time
- Staining of the inferior palpebral conjunctiva
- Epithelial defect—usually oval and in the central/superior cornea
- Defect surrounded by a rim of loose epithelium
- Edges may become smooth and rolled
- Stromal swelling with folds in the Descemet’s membrane
- Stromal lysis/melting
- May result in perforation
Diagnosis
Diagnosis of NK includes history, clinical examination, and diagnostic tests2,9
An assessment of etiologies is part of the identification process of NK1
- Post herpetic infection (HZV/HSV)
- Chronic ocular surface inflammation or injury (eg, dry eye disease)
- Contact lens wear
- Ocular surgery
- Topical ophthalmic drug toxicity
- Diabetes
- Stroke
- Multiple sclerosis
A complete slit lamp examination allows for evaluation of corneal and conjunctival changes2,9
Assessment of corneal sensation is a fundamental part of the diagnosis of NK2